
Introduction
Obtaining Import License for Clinical Trials
Clinical Trial Import License can be defined as an application requesting a license to allow import or manufacturing of any products for the sole purpose of clinical trials. If you’re also looking to manufacture products for clinical trial purposes, then this article will be a treat for you.
Before developing new medicine and marketing it to the public, it’s compulsory to seek a clinical trials license. In these trials, the efficacy, side effects, and quality of medicine are assessed to check whether it is safe to market.
Though the products manufactured to be used in clinical trials do not have to be registered by NPRA for this, still, it is very crucial to understand the regulatory framework and monitoring protocols concerning the rules of NPRA clinical trials.
NPRA, or National Pharmaceutical Regulatory Agency, is a Malaysian Ministry of Health governance body that regulates clinical trials and is responsible for issuing Clinical Trial Import Licenses in Malaysia.
Victory Pharma Consultancy
How NPRA Defines Clinical Trials
Clinical Trials are a process to determine the effectiveness and dangers of new medicines and other related medical practices for targeted patients.
The clinical trials are divided into four different clinical phases.
How to Cover These Phases
Before you apply to seek a clinical trial license for your products in Malaysia, there are some bases you need to cover.
The ministries of health and NPRA have created strict rules to ensure a proper check and balance for allowing clinical trials in Malaysia. You can’t just import a product or manufacture one and start its clinical trial.
You have to follow every single phase but first, apply for a clinical trial license from NPRA. NPRA sets some mandatory protocols that you have to follow to get a clinical trial import license in Malaysia.
If you don’t follow these different protocols, then you will not be considered eligible to conduct clinical trials for any drugs or medicines. If you also wish to conduct clinical trials and are looking for some information, don’t worry. In this article, we’ll discuss all the information related to clinical trials license in Malaysia.
So if you are unaware of the guidelines regarding clinical trials set forth by NPRA, then don’t worry; Victory Pharma Consultancy has got you covered.



Clinical Trial
Processing Time
Approximately 30 – 60 working days depending on completeness and complexity.

Why Choose Victory Pharma Consultancy
“We navigate the complexities of clinical trials—from regulatory submissions to operational coordination—ensuring compliance, efficiency, and results you can trust.”
We cover the following:
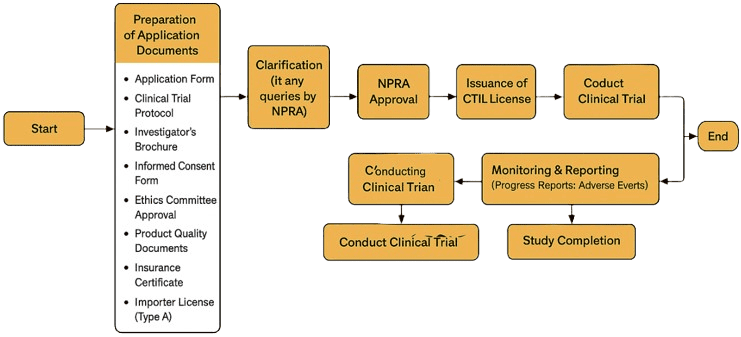
Preparation of Dossier
Compile all required documents following NPRA format.
Submission to NPRA
Submit through NPRA online system (QUEST system) or via manual submission depending on NPRA’s latest practice.
Evaluation by NPRA
NPRA evaluates safety, quality, and ethics committee compliance.
Approval and Issuance
Once approved, NPRA issues the CTIL which allows importation of clinical trial materials.
Drug Importation Monitoring
The hardcopy of permits are used to import IP’s in which we will liaise with the forwarding agent and maintain a record.
Clinical Trial
Frequently Asked Questions
What is CTIL?
What are the types of products that require CTIL?
- A product including placebo that is not registered with the Drug Control Authority (DCA) and intended to be imported for clinical trial purpose
- A product with a marketing authorization when used/formulated/packaged in a way different from the approved form; AND when used for unapproved indication to gain further information about an approved use for clinical trial purpose
- A traditional product with a marketing authorization with an indication for “traditionally used” when used for unapproved indication/therapeutic claims for clinical trial purpose
- An unregistered product, including placebo manufactured locally for clinical trial purpose
What are First-in-Human (FIH) clinical trials?
- They refer to trials when a new active substance under development is administered to a human for the first time. For FIH clinical trials, only an investigational product (IP) involving a new chemical entity/herbal/natural product with therapeutic claims will be accepted.
- Testing for these categories of products will NOT be considered as FIH clinical trials: (1) Generic product; (2) Registered traditional (herbal) product with an indication for “traditionally used” when being tested for therapeutic claims.
What is the pre-requisite to applying for CTIL?
Who can apply for CTIL?
How long does it take for the application to be reviewed and the license issued?
What does CTIL allow?
- Allows import of investigational product (IP) into Malaysia.
- Allows storage, handling, and usage for the clinical trial only.
- Does NOT allow commercial sale or marketing of the product.
